Glucose Anhydrous
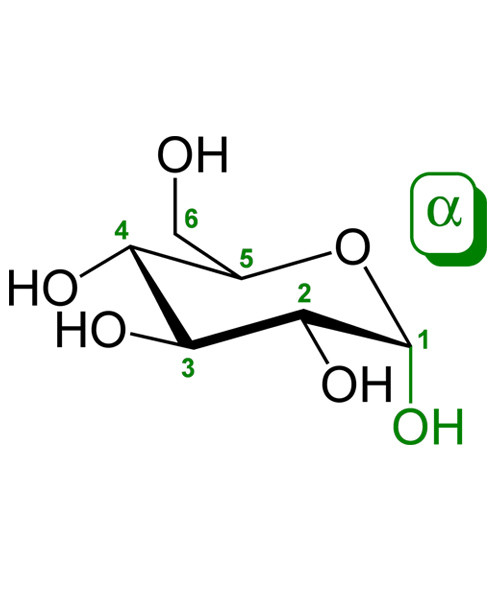
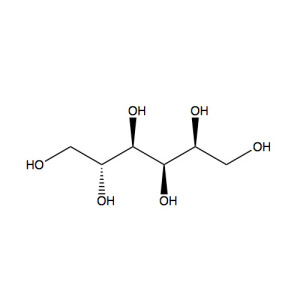
Glucose is a sugar with the molecular formula C6H12O6. The name “glucose” (/ˈɡluːkoʊs/) comes from the Greek word γλυκος, meaning “sweet wine, must”.[3] The suffix “-ose” is a chemical classifier, denoting a carbohydrate. It is also known as grape sugar. With 6 carbon atoms, it is classed as a hexose, a sub-category of monosaccharides. α-D-glucose is one of the 16 aldose stereoisomers. The D-isomer (D-glucose), also known as dextrose, occurs widely in nature, but the L-isomer (L-glucose) does not. Glucose is made during photosynthesis from water and carbon dioxide, using energy from sunlight. The reverse of the photosynthesis reaction, which releases this energy, is a very important source of power for cellular respiration. Glucose is stored as a polymer, in plants as starch and in animals as glycogen. Glucose can be obtained by hydrolysis of carbohydrates such as milk, cane sugar, maltose, cellulose, glycogen etc. It is however, manufactured by hydrolysis of cornstarch by steaming and diluting acid.[4]

Starting at
Product Description
| Preferred IUPAC name
D-Glucose
|
|
| Systematic IUPAC name
(2R,3S,4R,5R)-2,3,4,5,6-Pentahydroxyhexanal
|
|
| Other names
Blood sugar
Dextrose Corn sugar D-Glucose Grape sugar |
|
CAS Number
|
50-99-7 |
| 3DMet | B04623 |
| Abbreviations | Glc |
|
Beilstein Reference
|
1281604 |
| ChEBI | CHEBI:4167 |
| ChEMBL | ChEMBL1222250 |
| ChemSpider | 5589 |
| EC Number | 200-075-1 |
|
Gmelin Reference
|
83256 |
|
IUPHAR/BPS
|
4536 |
| Jmol interactive 3D | Image Image |
| KEGG | C00031 |
| MeSH | Glucose |
| PubChem | 5793 |
| RTECS number | LZ6600000 |
| UNII | 5SL0G7R0OK |
|
InChI[show]
|
|
|
SMILES[show]
|
|
|
Chemical formula
|
C6H12O6 |
| Molar mass | 180.16 g·mol−1 |
| Appearance | White powder |
| Density | 1.54 g/cm3 |
| Melting point | α-D-glucose: 146 °C (295 °F; 419 K) β-D-glucose: 150 °C (302 °F; 423 K) |
|
Solubility in water
|
909 g/1 L (25 °C (77 °F)) |
|
Dipole moment
|
8.6827 |
|
Specific
heat capacity (C) |
218.6 J K−1 mol−1[1] |
| Std molar entropy (So298) |
209.2 J K−1 mol−1[1] |
|
Std enthalpy of
formation (ΔfHo298) |
−1271 kJ/mol [2] |
|
Std enthalpy of
combustion (ΔcHo298) |
−2805 kJ/mol |
| Pharmacology | |
|---|---|
| ATC code | B05CX01 V04CA02, V06DC01 |
| Safety data sheet | ICSC 0865 |
| NFPA 704 |  |