Welcome visitor you can
login or register
0 items - $0.00
No products in the cart.
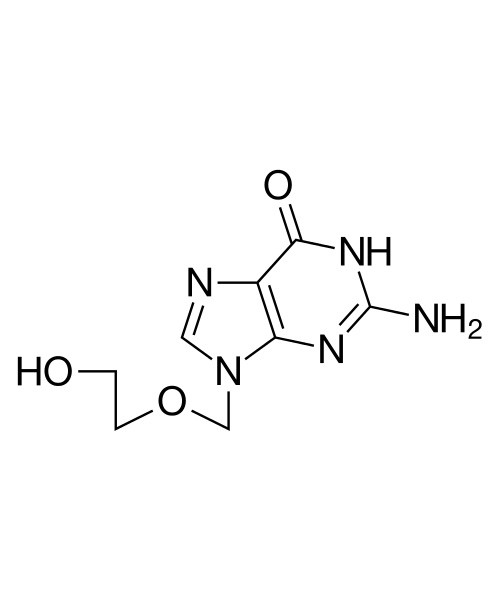
Aciclovir
Aciclovir (ACV), also known as acyclovir and acycloguanosine, is an antiviral medication.[3] It is primarily used for the treatment of herpes simplex virus infections, chickenpox, and shingles. Other uses include prevention of cytomegalovirus infections following transplant and infections due to Epstein-Barr virus. It is available by mouth and intravenously.[4]

Make an enquiry for this product
Category: Active Pharmaceutical Ingredients
Starting at
Product Description
| 2-Amino-1,9-dihydro-9-((2-hydroxyethoxy)methyl)-6H-purin-6-one |
| Pronunciation | /eɪˈsaɪkloʊvɪər/ |
| Trade names | Zovirax, others[1] |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a681045 |
| Licence data | US FDA:link |
| Pregnancy category |
AU: B3 |
| Legal status |
AU: S4 (Prescription only) for tablet and injection. Unscheduled for cream form under 10 g. CA: ℞-only UK: POM (Prescription only) for tablet and injection. GSL (OTC) for cream form under 2 g. US: ℞-only |
| Routes of administration |
Intravenous, oral, topical (including eye ointment) |
| Bioavailability | 15–20% (oral)[2] |
| Protein binding | 9–33%[2] |
| Metabolism | Hepatic |
| Biological half-life | 2-4 hours |
| Excretion | Renal (62-90% as unchanged drug) |
| CAS Number | 59277-89-3 |
| ATC code | J05AB01 D06BB03 S01AD03 |
| PubChem | CID: 2022 |
| IUPHAR/BPS | 4829 |
| DrugBank | DB00787 |
| ChemSpider | 1945 |
| UNII | X4HES1O11F |
| KEGG | D00222 |
| ChEBI | CHEBI:2453 |
| ChEMBL | CHEMBL184 |
| Synonyms | acycloguanosine |
| PDB ligand ID | AC2 (PDBe, RCSB PDB) |
| Formula | C8H11N5O3 |
| Molecular mass | 225.21 g·mol−1 |
|
SMILES[show] |
|
|
InChI[show] |
|
| Melting point | 256.5 °C (493.7 °F) |