Welcome visitor you can
login or register
0 items - $0.00
No products in the cart.
Ketoconazole
Ketoconazole (/ˌkiːtoʊˈkoʊnəˌzoʊl, -zɒl/[1][2]) (INN, USAN, BAN, JAN) is a synthetic imidazole antifungal drug used primarily to treat fungal infections. Ketoconazole is sold commercially as a tablet for oral administration (although this use has been discontinued in a number of countries), and in a variety of formulations for topical administration, such as creams (used to treat tinea; cutaneous candidiasis, including candidal paronychia; and pityriasis versicolor) and shampoos (used primarily to treat dandruff—seborrhoeic dermatitis of the scalp).[3]

Make an enquiry for this product
Category: Active Pharmaceutical Ingredients
Starting at
Product Description
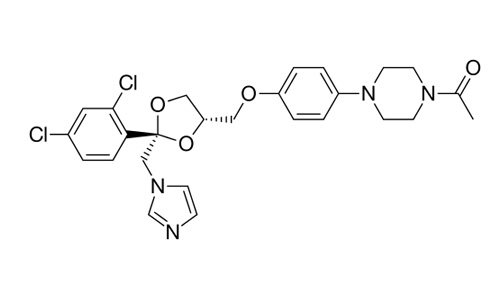
| 1-[4-(4-{[(2R,4S)-2-(2,4-Dichlorophenyl)-2-(1H-imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy}phenyl)piperazin-1-yl]ethan-1-one |
| Trade names | Nizoral |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a682816 |
| Licence data | US FDA:link |
| Pregnancy category |
AU : B3 US : C (Risk not ruled out) |
| Legal status |
UK: POM (Prescription only) US: OTC |
| Routes of administration |
Oral, topical |
| Bioavailability | Variable |
| Protein binding | 84 to 99% |
| Metabolism | Hepatic |
| Biological half-life | Biphasic |
| Excretion | Biliary and renal |
| CAS Number | 65277-42-1 |
| ATC code | J02AB02 D01AC08 G01AF11 |
| PubChem | CID: 456201 |
| IUPHAR/BPS | 2568 |
| DrugBank | DB01026 |
| ChemSpider | 401695 |
| UNII | R9400W927I |
| KEGG | D00351 |
| ChEBI | CHEBI:48336 |
| ChEMBL | CHEMBL75 |
| PDB ligand ID | KTN (PDBe, RCSB PDB) |
| Formula | C26H28Cl2N4O4 |
|---|---|
| Molecular mass | 531.431 g/mol |
| SMILES[show] | |
| >InChI[show] | |