Welcome visitor you can
login or register
0 items - $0.00
No products in the cart.
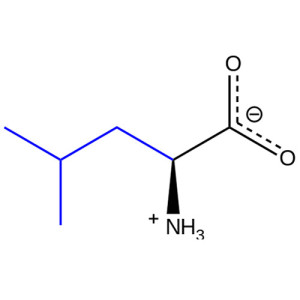
L-Isoleucine
Isoleucine (abbreviated as Ile or I) encoded by the codons AUU, AUC, and AUA is an ɑ-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated -+NH3 form under biological conditions), an α-carboxylic acid group (which is in the deprotonated –COO- form under biological conditions), and a hydrocarbon side chain, classifying it as a non-polar, uncharged(at physiological pH), aliphatic amino acid. It is essential in humans, meaning the body cannot synthesize it, and must be ingested in our diet. Isoleucine is synthesized from pyruvate employing leucine biosynthesis enzymes in other organisms such as bacteria.[1]
SKU: n/a.
Category: Amino Acids
Starting at $30.10
Product Description
| IUPAC name
Isoleucine
|
|
| Other names
2-Amino-3-methylpentanoic acid
|
|
CAS Number
|
73-32-5 |
| ChEBI | CHEBI:58045 |
| ChemSpider | 6067 |
| DrugBank | DB00167 |
|
IUPHAR/BPS
|
3311 |
| Jmol interactive 3D | Image |
| KEGG | D00065 |
| PubChem | 791 |
| UNII | 04Y7590D77 |
|
InChI[show]
|
|
|
SMILES[show]
|
|
|
Chemical formula
|
C6H13NO2 |
| Molar mass | 131.18 g·mol−1 |
|
Structure and
properties |
Refractive index (n), Dielectric constant (εr), etc. |
|
Thermodynamic
data |
Phase behaviour solid–liquid–gas |
|
Spectral data
|
UV, IR, NMR, MS |