0 Carrito - $0.00
No products in the cart.
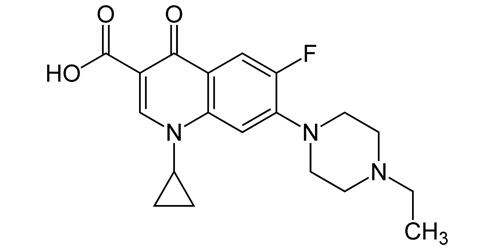
ENROFLOXACINA BASE
Enrofloxacin (ENR) is a fluoroquinolone antibiotic sold by the Bayer Corporation under the trade name Baytril. Enrofloxacin is currently approved by the FDA for the treatment of individual pets and domestic animals in the United States. In September 2005, the FDA withdrew approval of Baytril for use in water to treat flocks of poultry, as this practice was noted to promote the evolution of fluoroquinolone-resistant strains of the bacterium Campylobacter, a human pathogen.[4]

Realizar una consulta de este producto
Category: INGREDIENTES FARMACÉUTICOS ACTIVOS
Starting at
Product added!
Add to wishlist
The product is already in the wishlist!
Add to wishlist
Product Description
| 1-cyclopropyl-7-(4-ethylpiperazin-1-yl)-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
AU: B3 US: C (Risk not ruled out) |
| Legal status |
AU: S4 (Prescription only) UK: POM (Prescription only) |
| Routes of administration |
Oral, subcutaneous injection, topical (ear drops) |
| Bioavailability | 80% in dogs, 65-75% in sheep [1] |
| Metabolism | Renal and non-renal[2] |
| Biological half-life | 4–5 hours in dogs, 6 hours in cats, 1.5 – 4.5 hours in sheep. |
| Excretion | Bile (70%); Renal (30%)[3] |
| CAS Number | 93106-60-6 |
| ATCvet code | QJ01MA90 |
| PubChem | CID: 71188 |
| ChemSpider | 64326 |
| UNII | 3DX3XEK1BN |
| KEGG | D02473 |
| ChEBI | CHEBI:35720 |
| ChEMBL | CHEMBL15511 |
| Formula | C19H22FN3O3 |
| Molecular mass | 359.4 |
| SMILES[show] | |
| InChI[show] | |
Be the first to review “ENROFLOXACINA BASE” Cancel reply
Related Products

Product added!
Add to wishlist
The product is already in the wishlist!
Add to wishlist
HIERRO DEXTRAN 10%
Product added!
Add to wishlist
The product is already in the wishlist!
Add to wishlist
GUAIACOL
Product added!
Add to wishlist
The product is already in the wishlist!
Add to wishlist
QUINFAMIDA MICRONIZADA

Product added!
Add to wishlist
The product is already in the wishlist!
Add to wishlist

Reviews
There are no reviews yet.