Welcome visitor you can
login or register
0 items - $0.00
No products in the cart.
L-Aspartic acid
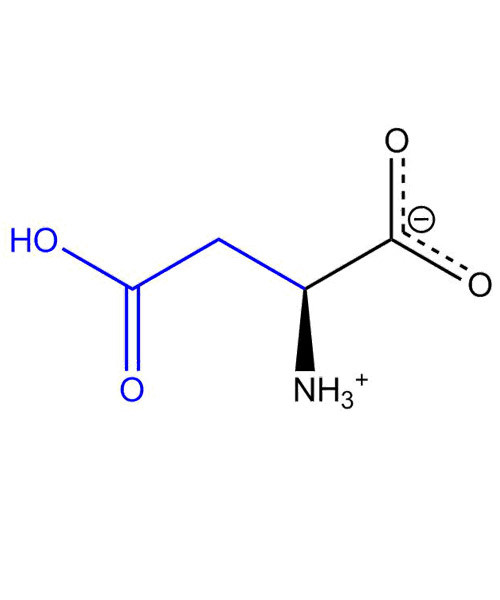
Aspartic acid (abbreviated as Asp or D; encoded by the codons [GAU and GAC]) is an ɑ-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated -+NH3 form under biological conditions), an α-carboxylic acid group (which is in the deprotonated –COO- form under biological conditions), and a side chain CH2COOH, classifying it as a charged(at physiological pH), aliphatic amino acid. It is semi-essential in humans, meaning the body can synthesize it from oxaloacetate.
SKU: n/a.
Category: Amino Acids
Starting at $20.30
Product Description
| IUPAC names
Trivial: Aspartic acid
Systematic: 2-Aminobutanedioic acid |
|
| Other names
Aminosuccinic acid, asparagic acid, asparaginic acid[1]
|
|
CAS Number
|
617-45-8 56-84-8 (L-isomer) 1783-96-6 (D-isomer) |
| ChEBI | CHEBI:22660 |
| ChEMBL | ChEMBL139661 |
| ChemSpider | 411 |
| EC Number | 200-291-6 |
| Jmol interactive 3D | Image Image |
| KEGG | C16433 |
| PubChem | 424 |
| UNII | 28XF4669EP |
|
InChI[show]
|
|
|
SMILES[show]
|
|
|
Chemical formula
|
C4H7NO4 |
| Molar mass | 133.10 g·mol−1 |
| Appearance | colourless crystals |
| Density | 1.7 g/cm3 |
| Melting point | 270 °C (518 °F; 543 K) |
| Boiling point | 324 °C (615 °F; 597 K) (decomposes) |
|
Solubility in water
|
4.5 g/L[2] |
| Acidity (pKa) | 3.9 |
| Safety data sheet | See: data page |
| NFPA 704 | 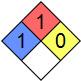 |
|
Structure and
properties |
Refractive index (n), Dielectric constant (εr), etc. |
|
Thermodynamic
data |
Phase behaviour solid–liquid–gas |
|
Spectral data
|
UV, IR, NMR, MS |